

Hydrogen sulfide (H2S) is often identified as a potential culprit of odors and nuisance complaints near municipal solid waste (MSW) landfills. Some base their complaints on information found on the Internet as fact. As experts, let’s start by saying data from other landfills or pulled from an AI browser summary online will not provide accurate answers. H2S concentrations vary widely and are unique to individual landfills.
How is H2S generated in an MSW landfill, and why do concentrations vary?
Calcium sulfate (CaSO4•2H2O, aka gypsum), the primary ingredient in wallboard (aka drywall), can be biologically converted to H2S under select and somewhat rare conditions. Specifically, seven conditions are required for the biodegradation of gypsum to H2S. See (Gypsum Association, Industry Technical Paper: Treatment and Disposal of Gypsum Board Waste (Jan. 1991); Gypsum Association, Treatment and Disposal of Gypsum Board Waste, Part II, Technical Paper (Mar. 1992).
Condition 1 – Liquid Water. The biological conversion of sulfate to H2S occurs in the aqueous phase—i.e., sufficient free liquids must be present, and sulfates must dissolve into the free liquids. Modern landfills with leachate collection systems may experience intermittent perched and discrete zones of saturation within the waste mass, particularly following periods of extended precipitation. Low-permeability confining layers (e.g., clay or clay-like soil used for intermediate cover) may temporarily trap water/leachate in discrete pockets within the landfill.
Condition 2 – Source of Soluble Sulfate. Gypsum, having the chemical formula CaSO4•2H2O, is a source of soluble sulfate. Gypsum sources include wallboard (aka drywall), flue gas desulfurization (FGD) material from coal-fired power plants, and some industrial wastes. Sulfates and sulfur compounds can also be present in lower concentrations in other waste streams, depending on what the MSW landfill accepts.
Condition 3 – Sulfate-reducing Bacteria. Sulfate-Reducing Bacteria (“SRB”) use dissolved sulfate as an electron acceptor in the oxidation of carbon. Primary SRB include Desulfovibrio and Desulfotomaculum. These SRBs, as well as many other bacteria, are commonly present in MSW landfills. However, the presence of SRB within a landfill may not be ubiquitous, and may be limited to regions where the other required conditions favor their existence and survival.
Condition 4 – Organic Material. SRBs use organic material as a food source to multiply and degrade sulfate to H2S. Carbon serves as a source of energy for the bacteria. Typical MSW has a high organic content due to a wide variety of organic materials such as wood, paper, cardboard, food, vegetative waste, and fabrics. Many communities with recycling programs help divert these waste materials for reuse and recycling.
Condition 5 – Anoxic Environment. SRBs thrive under anoxic (without oxygen) conditions. The presence of oxygen can kill SRBs. While anoxic conditions are typically not present in areas where MSW was recently disposed, they are typical in portions of MSW landfills where organic wastes have been present for at least a few months and decompose to produce methane and carbon dioxide.
Condition 6 – Appropriate pH Range. SRB reduction of sulfate to H2S is reportedly optimum within a pH range of about 7 to 8, and does not occur outside a pH range of about 4 to 9. The pH range within a typical MSW landfill falls within this activity range.
Condition 7 – Appropriate Temperature Range. SRB reproduction and H2S generation are reportedly optimum within a range of about 30 °C to 38 °C (86 °F to 100 °F). Many MSW landfills are within or a little above this optimum range. Studies of SRB in geologic environmental settings found reduced activity above about 60 °C (140 °F), and no activity above about 80 °C (176 °F). Similarly, SRB activity ceases in freezing conditions.
In summary, although the necessary conditions for H2S generation are likely intermittently present within some discrete pockets within many MSW landfills, the conditions are not likely ubiquitous throughout the waste. MSW landfill conditions and waste composition are typically highly heterogeneous with respect to both location within the landfill and time. Thus, there are zones within landfills where many, but not all of the seven required conditions are present, and H2S generation does not occur. For example, there are undoubtedly many regions within landfills where free liquids (i.e., saturated conditions) are not present and, therefore, SRB conversion of sulfates to H2S does not occur, despite the presence of the other six conditions.
Similarly, a landfill may have pockets where bulk sulfate-containing waste has been disposed of but where the internal portion of the pocket is not exposed to moisture, organics, or SRB—each a necessary condition for converting sulfate to H2S.
Considering these seven conditions and heterogeneous landfill conditions, there are too many variables to provide a reliable and defendable quantitative model for H2S generation at all MSW landfills.
Monitoring and Treating Landfill H2S Conditions
We invite you to use our free resource library to learn more about how monitoring and data collection can protect your workers and the surrounding environment.

Hydrogen sulfide (H2S) levels are creeping up at some landfills, especially those that take C&D waste; some are seeing concentrations in the thousands to 30,000 parts per million (up from about 20 to 40 ppm ten years ago). And even at very low concentrations, H2S can be problematic.
Material Recovery Facility residuals, which typically contain significant amounts of pulverized drywall, are high in gypsum and sulfate. Once broken down, residuals become a high-surface-area material, leaching into and spreading through waste. When reacting with water and organics, it can potentially generate H2S. With a drive to divert more C&D debris, and regulations tightening around H2S, operators’ jobs get harder as they work to stave off emissions from this corrosive, flammable compound notorious for its “rotten egg” odor.
When building out their gas collection systems, controlling H2S becomes even more daunting. Sol Sim, an SCS Engineers Vice President, explains, “We see H2S concentrations jump when we expand landfill gas collection systems, often in cells that contain C&D residual screening materials. The gas was there all along but sequestered. Now it’s coming out of the ground, and the onset of issues can spike suddenly.”
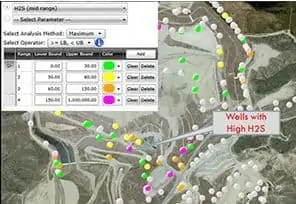
When spikes come on quickly, Sim’s team implements turnkey interim treatment approaches. They start by identifying the gas collection wells with the highest contributors and act fast to get them into compliance.
SCS teams take a two-pronged approach by stepping back and thinking about the big picture while taking action. It provides a major advantage to moving too quickly.
The more data, the better. Your engineers can simulate treatment with various media to assess the impact on flare inlet concentrations. And knowing potential impact at the flare is critical; it’s the compliance point where regulators measure sulfur dioxide (SO2) emissions.
SO2 can’t be controlled through combustion, so removing H2S from waste before sending gas to the flare is essential. Sim thinks back to problems he’s investigated for clients who had SO2 sneak up on them, causing failed sulfur dioxide emissions testing.
The proactive measure of identifying problematic gas wells and treating them is key to staying in compliance. And Sim often finds clients using interim solutions as long as they can. He has seen them work well for up to five years but they don’t resolve operators’ long-term issues, which will become more challenging as our waste streams continue to change or as landfills continue to accept more and more C&D materials.

“We’re going to investigate thoroughly to pinpoint and understand the cause, but we do take immediate action in the interim. As part of the solution, we’ll develop an informed strategy to prevent issues well into the future,” he says.
For the longer haul, it takes time to get building permits. Coming up with permanent engineering designs and treatments requires a lot of troubleshooting and research. Even once engineers identify a lasting fix, it takes time to manufacture and install larger vessels and other infrastructure.
But they don’t wait for all these pieces to come together to act.
The priority is getting operators in compliance right away or taking down emissions if they are on the verge of noncompliance. As work begins, operators can breathe a little easier knowing they have time to figure out how to allocate resources and funds to implement a more permanent strategy.
“We’ve seen where data we’ve gathered while working on the immediate problem enables our clients to gain insight to make good decisions around rightsizing their infrastructure moving forward,” Sim says.
Watch and study while addressing the immediate problem.
Sim emphasizes that operators should not be surprised or act too quickly when they turn on the gas extraction system and see spikes in H2S concentrations. There is usually an initial spike from a new high H2S producing area at the onset of gas collection. He has seen operators abruptly stop extracting, which can lead to odors or other compliance issues.
“When you put in a treatment system, you can take out the initial surge in H2S to allow time for the concentrations to level out. It’s important to allow that window for initial surges to run their course to understand the problem better. Otherwise, you could over-design your system around a short-term event,” Sim advises.
He points to a real-life scenario: a site that skipped the interim step of starting with a less expensive initial solution. Once they started drawing on the gas, they realized the problem was not as substantial as originally thought, and they didn’t need a multi-million-dollar system in the end.
A balancing act.
“Imagine H2S generation as an expanding balloon; if you pop it, air rushes out fast [akin to when you first pull gas from the ground]. That concentration level scares people. But if you react by shutting off extraction points, your balloon will continue to expand and eventually create odor problems. The goal is to extract the gas and H2S at the rate it is being generated, so it’s a balancing act, where expertise and technology both play key roles,” Sim says.
Early work typically begins by identifying wells that are the highest contributors and concentrating efforts there. It’s a complex process as sites can have fifty to thousands of collection points. Having the historical data and saving the data to watch the trend makes identifying and analyzing specific wells or clusters much more efficient.
Successfully attacking those high offenders requires an understanding of flow and concentrations. After locating the problem area, Sim takes samples using Dräger tubes at strategic points throughout individual wells and headers to identify concentrations. Gas well monitoring and the corresponding flow data will tell you if you’ve taken emissions down sufficiently.
More Resources:
SCSeTools Landfill Data Monitoring and Analysis
Staying Ahead of Odor Management at Solid Waste Facilities