

SCS Engineers’ Gomathy Radhakrishna Iyer explains, “The structure of PFAs is a carbon and fluorine bond, and that bond is considered one of the strongest in nature. For industry, Chlorofluorocarbons (CFC), a volatile derivative of methane, ethane, and propane, creates problems globally after they’ve been released. Chlorofluorocarbons are strong greenhouse gases and are also responsible for the destruction of stratospheric ozone.
The most publicized of these compounds are those used as coolants in refrigeration and air conditioners, as propellants in spray cans and similar products, and as solvents for industrial purposes. Chlorofluorocarbons are far less abundant than carbon dioxide in the atmosphere. Still, they are 10,000 times more potent as a greenhouse gas and can remain in the atmosphere for more than 45 to 100 years. Reference
Iyer continues, “PFAS has the same kind of carbon-fluorine bond as CFC but linked to several C-F bonds like a chain making them even more inert and hard to degrade. Breaking this bond is what makes finding effective leachate treatments challenging, but certainly possible.”
It takes a savvy engineer to design safe and effective systems. We’re very proud of our Young Professionals like Gomathy – they’re smart and continue learning with the guidance of our VEPs – very experienced professionals.
Open positions at SCS Engineers for YPs and VEPs
Introduction
PFAS are a class of synthetic fluorinated chemicals used in many industrial and consumer products, including defense‐related applications. They are persistent, found at low levels in the environment, and bio‐accumulate. Studies have shown these compounds being detected more often in surface water, sediments and/or bioaccumulated into fish tissue. Because of the greater affinity of longer chain per‐ and polyfluoroalkyl substances (PFASs) compounds for fish than other environmental matrices, certain compounds are often found in fish tissue, but not in the water or sediment. Table 1 shows average concentrations of PFOA and PFOS in landfill leachates around the world. The USEPA health advisory level is 70 ppt for PFOA and PFOS.
Table 1. Concentrations of PFAS compounds in Landfill Leachate around the world
| Compound | US | Germany | China |
| PFOA (ppt) | 660 | 150 | 280-214,000 |
| PFOS (ppt) | 110 | 30 | 1,100-6,000 |
Treatment Options for PFOS and PFOA
The removal of PFASs from drinking water has been the USEPA’s national priority. Recent discoveries of PFAS/PFOS in drinking water in multiple states in the US has heightened interest in these emerging contaminants. Federal, state, and local agencies are formulating regulatory limits that vary greatly. These limits seem to be centered on drinking water, but these developments are driving disposal of existing stores of chemicals containing PFAS/PFOS and environmental media contaminated with PFAS/PFOS
Treatment processes that can remove PFAS chemicals from drinking water may include high-pressure membrane systems such as RO, granular activated carbon (GAC), or ion exchange as shown in Figure 1. The more conventional water treatment technologies such as (e.g., aeration) are not typically effective.
Figure 1. PFAS Removal Processes (a) Membranes, (b) GAC and (c) Ion Exchange Resins
Landfill Leachate RO Treatment Plant – New Hanover County, North Carolina
New Hanover County upgraded its leachate treatment system to meet stricter regulatory standards for surface water discharges, particularly standards relating to metals (arsenic) and ammonia. Sampling by NC DEQ showed the new RO plant is filtering out PFAS. Table 2 shows the results from February 2019.
Figure 2. New Hanover County Leachate and PFAS Treatment Plant
Table 2. Concentrations of PFAS compounds in Leachate at New Hanover County Landfill
| PFAS Constituent | Raw | Treated | Surface water |
| PFOA (ppt) | 1,250 | < 0.6 | 3.9 |
| PFOS (ppt) | 228 | < 0.6 | 7.1 |
Comparison of GAC Types for PFOA and PFOS Removal
Four different types of GAC, i.e., Re-agglomerated Bituminous, Lignite, Enhanced Coconut and Enhanced Coconut (Blend) were evaluated under identical operating conditions and influent water quality. Figure 4 shows results from these four GAC products for PFOA/PFOS removal vs time.
Figure 4. GAC Treatability study for removal of PFOA and PFOS
Re-agglomerated bituminous coal GAC (FILTRASORB) significantly outperformed: Lignite, Enhanced Coconut and Enhanced Coconut (Blend).
Summary:
PFAS compounds are of concern because they do not break down in the environment, bioaccumulate in humans and biota, and may pose risks to human health
GAC, Synthetic adsorbent, and ion exchange resins are widely used for PFAS removal. Capacity and leakage of PFASs into the treated water varies depending on the specific PFASs, the type of adsorbent used.
PFAS removal may be influenced by pH, water temperature, contact time, Natural Organic Matter, and chlorine. For complete PFAS removal, a polishing may be required.
Disposal methods for PFAS waste streams include high-temperature incineration or landfilling. Landfilling is not favored since the PFAS load would increase, and many landfills will not accept PFAS waste.

Per- and poly-fluoroalkyl substances (PFAS) are receiving increasing attention from regulators and the media. Within this large group of compounds, much of the focus has been on two long-chain compounds that are non-biodegradable in the environment: PFOS (perfluorooctane sulfonate) and PFOA (perfluorooctanoic acid).
Long detected in most people’s bodies, research now shows how “forever chemicals” like PFAS accumulate and can take years to leave. Scientists have even tracked them in biosolids and leafy greens like kale. Recent studies have linked widely used PFAS, including the varieties called PFOA and PFOS, to reduced immune response and cancer. PFAS have been used in coatings for textiles, paper products, cookware, to create some firefighting foams and in many other applications.
Testing of large public water systems across the country in 2013 through 2015 found PFAS detected in approximately 4 percent of the water systems, with concentrations above the USEPA drinking water health advisory level (70 parts per trillion) in approximately 1 percent (from ITRC Fact Sheet). Sources of higher concentrations have included industrial sites and locations were aqueous film-forming foam (AFFF) containing PFAS has been repeatedly used for fire fighting or training. Source identification is more difficult for more widespread low-level PFAS levels.
With the EPA positioned to take serious action on PFAS in 2020 and beyond, regulators in many states have already started to implement their own measures, while state and federal courts are beginning to address legal issues surrounding this emerging contaminant. State actions have resulted in a variety of state groundwater standards for specific PFAS compounds, including some that are significantly lower than the USEPA advisory levels. These changes mean new potential liabilities and consequences for organizations that manufacture, use, or sell PFAS or PFAS-containing products, and also for the current owners of properties affected by historic PFAS use. If you operate a landfill or own a site with PFAS history this may be something you need to discuss and plan now.
Questions for property owners, property purchasers, and manufacturers include:
If PFAS treatment or remediation is required, a number of established options to remove PFAS from contaminated soil and groundwater are available, including activated carbon, ion exchange or high-pressure membrane systems. On-site treatment options, including in-situ or ex-situ alternatives, the management of reject streams with concentrated PFAS waste where applicable, are also available.
Do You Need Help?
Need assistance with PFAS or have an idea that you would like to discuss? Contact , or find the SCS Engineers location nearest you.
Per- and poly-fluoroalkyl substances (PFAS) are receiving increasing attention from regulators and the media. Within this large group of compounds, much of the focus has been on two long-chain compounds that are non-biodegradable in the environment: PFOS (perfluorooctane sulfonate) and PFOA (perfluorooctanoic acid). Long detected in most people’s bodies, research now shows how “forever chemicals” like PFAS accumulate and can take years to leave. They persist even when excreted through urine. Scientists have even tracked them in biosolids and leafy greens like kale. Recent studies have linked widely used PFAS, including the varieties called PFOA and PFOS, to reduced immune response and cancer. PFAS have been used in coatings for textiles, paper products, cookware, to create some firefighting foams and in many other applications.
Testing of large public water systems across the country in 2013 through 2015 found PFAS detected in approximately 4 percent of the water systems, with concentrations above the USEPA drinking water health advisory level (70 parts per trillion) in approximately 1 percent (from ITRC Fact Sheet.) Sources of higher concentrations have included industrial sites and locations were aqueous film-forming foam (AFFF) containing PFAS has been repeatedly used for fire fighting or training.
Source identification is more difficult for more widespread low-level PFAS levels. For example, in Madison, Wisconsin, PFAS have been detected in 14 of 23 municipal water supply wells, but the detected concentrations were below the USEPA’s health advisory levels for PFOA and PFOS. A study of potential PFAS sources near two of the Madison wells identified factories, fire stations, landfills, and sludge from sewage treatment plants as possible sources, but did not identify a specific source.
With the EPA positioned to take serious action on PFAS in late 2019 and 2020, regulators in many states have already started to implement their own measures, while state and federal courts are beginning to address legal issues surrounding this emerging contaminant. State actions have resulted in a variety of state groundwater standards for specific PFAS compounds, including some that are significantly lower than the USEPA advisory levels. These changes mean new potential liabilities and consequences for organizations that manufacture, use, or sell PFAS or PFAS-containing products, and also for the current owners of properties affected by historic PFAS use.
Questions for manufacturers, property owners, and property purchasers include:
If remediation is required, a number of established options to remove PFAS from contaminated soil and groundwater are available, including activated carbon, ion exchange or high-pressure membrane systems. On-site treatment options, including the management of reject streams where applicable, are also available.
Do You Need Help?
Need assistance with PFAS or have an idea that you would like to discuss? Contact for more information.
Use these resources to explore more about PFAS each is linked to helpful articles and information.
We recommend reading this article series to stay abreast of relevant knowledge from Bryan Staley, president and CEO of the Environmental Research & Education Foundation (EREF); Anne Germain, vice president of technical and regulatory affairs for the National Waste & Recycling Association (NWRA); Viraj deSilva, SCS Engineers wastewater treatment director; and testing results from New Hanover County whose capital investment in landfill infrastructure has proven to successfully treat effluent water to meet higher standards.
Forester University recently hosted Dr. Viraj deSilva P.E., BCEE of SCS Engineers in their well-received educational webinar “All About PFAS: Emerging Contaminants That Are Everywhere.”
Dr. deSilva teaches you all you need to know to protect yourself and your community from PFAS—from generation, formation, and environmental release to sampling and analysis.
He provides an in-depth overview of the treatment of PFAS in sources that do not currently have maximum containment levels, such as landfill leachate, wastewater, surface water, and groundwater.
This course covers nomenclature, chemistry, sources, exposure, and future concerns as well as discusses the current regulatory status of these contaminants.
Learning Objectives
We encourage our readers to see the webinar on Forester University’s website. Credits: 1 PDH / 0.1 CEU. Forester offers registration savings to groups. Register here.
Additional Resources with Links – click to read
Managing landfill leachate and wastewater treatment are increasingly challenging and costly for landfill owners and operators. In some cases, publicly owned treatment works (POTWs) are required to impose limitations on liquids received at their facilities, resulting in increased charges, or the POTW could refuse to permit or process the leachate wastewater altogether. These developments are due in part to more stringent discharge requirements and the shift to newer disinfection technology that has limited the POTW’s ability to accept higher strength wastewaters. As a result, many facilities and landfill operators are facing higher costs and fewer options for disposal.
Another factor that affects landfills is the fact that the composition of leachate in landfills differs depending on the degree of leachate stabilization and a seasonal increase in quantity as well as on the influence of more frequent and higher intensity storms due to changing climatic conditions.
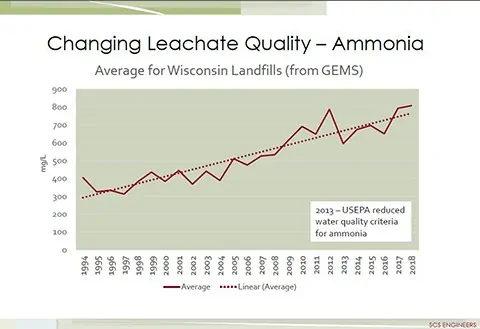
The single most influential factor on the volume of landfill leachate is precipitation. The most influential factor regarding leachate quality is that leachate typically contains high concentrations of organic compounds, ammonia and other forms of nitrogen, metals, and dissolved solids. Ammonia concentrations in the leachate, from many landfills, are increasing every year as shown in the graph below. Compounding the high strength concentrations of constituents found in landfill leachate are the emerging contaminants of concern including per and poly-fluoroalkyl substances (PFAS) that are now a significant concern with the U.S. EPA and many state environmental agencies.
Top 5 Questions and Answers When Selecting the Right Leachate Treatment Option for Your Landfill
Without considering leachate recirculation and a host of other factors, this blog provides answers to frequently asked questions regarding the analysis of treatment options for landfill leachate.
ONE: What is an example of a typical work scope of a leachate treatment options analysis?
TWO: What information is necessary to begin assessing the on-site treatment options of leachate?
THREE: What constituents should I expect to have analyzed to assess the options for leachate wastewater treatment?
FOUR: What are some of the issues taken into account regarding treating ammonia-N in leachate/wastewater?
FIVE: What are some examples of the options for how to effectively treat ammonia-N in leachate on-site?
We hope you find our SCS Advice from the Field blogs helpful. For more information, we recommend these articles and resources: